Challenge
Virtual screening is an important drug discovery method used in modern pharmacology. It is a tool that researchers use to find molecules that are the most aggressive against the target protein molecule (which is a part of the virus). Molecules with specific properties are chosen from a chemical database of existing agents. One of the desirable properties is the condition that the molecule does not protrude into specific regions (called sterically forbidden regions). The client suggested an algorithm for creating a shrink-wrapped surface to check whether the molecule has this property.
The shrink-wrapped surface method allows scientists to choose the molecular surface conformation with the smallest surface area by creating a van der Waals molecular surface model (conformation is the specific spatial orientation of atoms in a molecule).
Axmor software engineers were asked to create a computational tool that would take molecular data files in specific formats (mol, mol2, sdf, pdb), process them using a geometric structuring algorithm, and generate a surface file in obj format (a set of 3D-coordinates of vertices and edges). The tool also generates a file containing numerical characteristics of the results (e.g., the volumes of molecular interpenetration, which is a quantitative measure of the difference in the geometrical location of molecules). Additionally, the existing CDKPsearch package for virtual screening using the Kabsch algorithm was improved.
Solution
Axmor software engineers used an icosahedral mesh to create van der Waals surfaces of initial molecules and the shrink-wrapped surface. An icosahedral mesh is more uniform than the classical spherical one and is easier to integrate into an upper level.
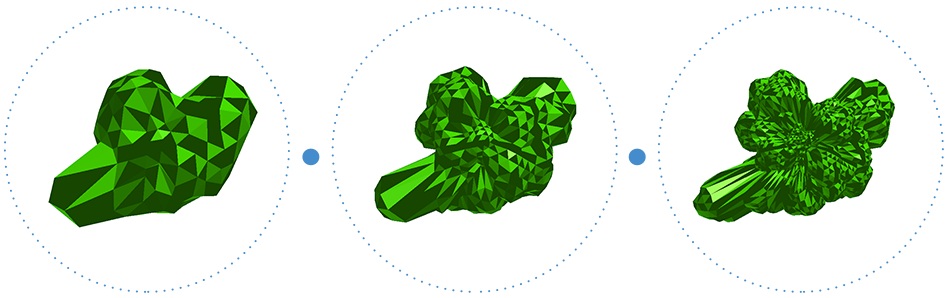
Fig. 1. An approximation of the molecular surface of ciclosporin:
162 vertices, 642 vertices, and 2,562 vertices
Fig. 2. Examples of icosahedral meshes
with an increasing number of vertices

The points for the molecules' atoms that are located at the nodes of spherical meshes (molecular points) are calculated.
For each molecular point, the nearest icosahedral point is found (the distance is measured as the angle between the radius vectors), and its spherical radius is taken as the maximum difference between the current value and the radius of the current molecular point.
The remaining “holes” are covered by approximating the radii of the nearest calculated directions. If there are enough molecular points relative to the dimensions of the icosahedral mesh (achieved by increasing the frequency of spherical meshes of atoms), we obtain a good approximation of the initial molecular surface.We then used the procedure for calculating volumes of molecular interpenetration, which consists of calculating the volume of the truncated tetrahedron (using a third-order determinant) and adding it up for all tetrahedrons.